Berkshire News
BPR Authorization for SatPax® 70/30 IPA Presaturated Wipes!
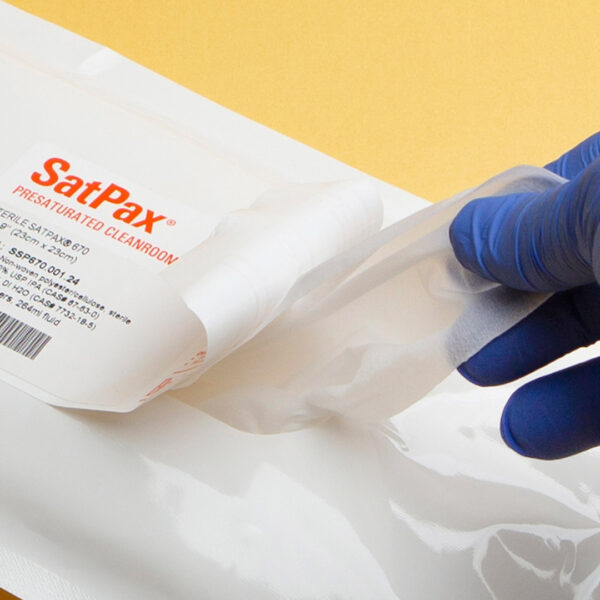
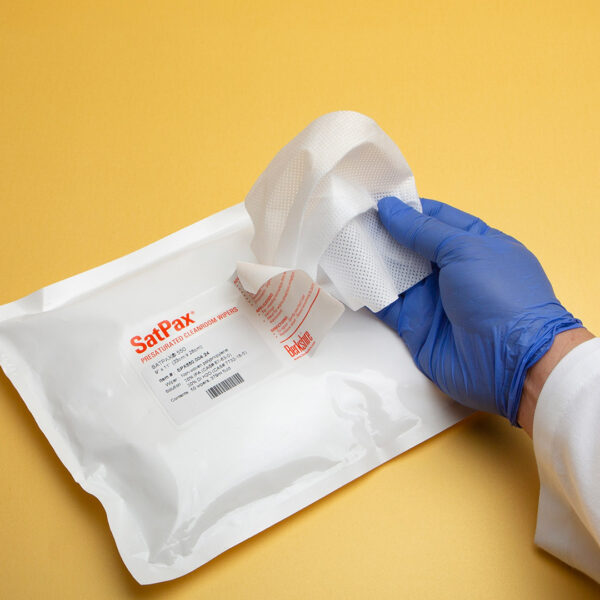
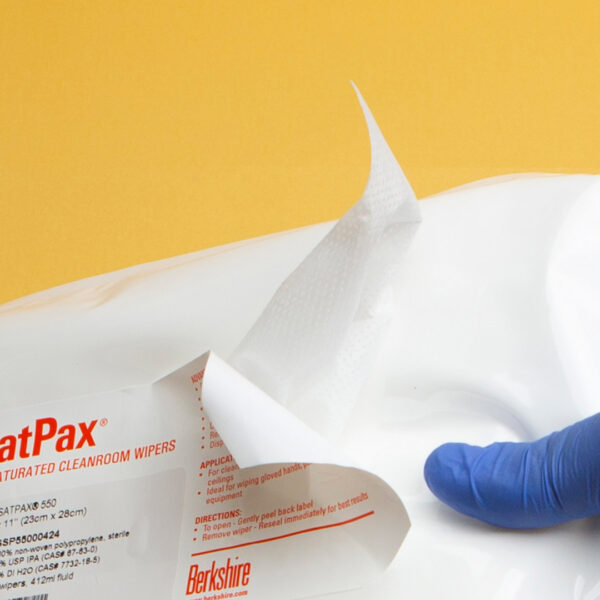
We are excited to announce that our SatPax® 70/30 IPA presaturated wipes have received Union Authorisation from the European Commission! These wipes, consisting of 70% USP Grade isopropyl alcohol (IPA) and 30% water for injection (WFI) or deionized (DI) water, are now approved for sale throughout the EU and EEA (European Economic Area).
What is Biocidal Products Regulation?
BPR, Regulation (EU) 528/2012, governs the availability and use of biocidal products in the market. Berkshire’s biocidal products help control unwanted microorganisms that can harm human or animal health or the environment through the action of their active substances. The BPR provides a harmonised and centralised authorisation and approval process for biocidal products at a European level.
Why Choose SatPax® 70/30 IPA Wipes?
Designed for efficiency and safety, our SatPax® wipes are available in both sterile and non-sterile options. They are ideal for various applications, ensuring a quick and effective solution for disinfecting surfaces and equipment.
Look for the BPR logo on the product listing to quickly identify our authorised biocidal products. Their authorisation number, EU-0032869-0000, further assures compliance with European safety and efficacy standards.
This achievement marks a significant milestone for us, reinforcing our commitment to delivering quality products that meet regulatory standards. Whether you’re in healthcare, laboratories, or any environment demanding high standards of cleanliness, SatPax® wipes are here to support your needs.
Stay tuned for more updates, and thank you for your continued support!